Identification and Implementation of Biocatalytic Transformations in Route Discovery: Synthesis of Chiral 1, 3-Substituted Cyclohexanone Building Blocks
| Products/Services Used |
Details |
Operation |
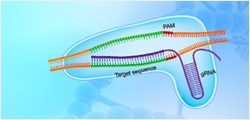
| Codon Optimization |
The genes encoding EREDs and nitrilases were codon optimised for expression in E. coli
BL21(DE3), synthesised, and cloned into pET28a at GenScript. Transformation, shake flask
enzyme expression and 50 L fermentation trials were performed as described previously |
Get A Quote |
Several biocatalytic approaches for the preparation of optically pure methyl 3
oxocyclohexanecarboxylates (S)-, (R)-1 and 3-oxocyclohexanecarbonitriles (S)-, (R)-2 have been
successfully demonstrated. Screening of reaction-focused enzyme collections was used to
identify initial hits using three enzymatic strategies. Reaction optimization and scale-up enabled
the production of chiral intermediates for route scouting efforts on scales of up to 100 g. The
enzymes applied in these processes (lipases, enoate reductases and nitrilases) have been shown to
be robust catalysts for drug manufacturing and represent a green alternative to conventional
methods to access these chiral cyclohexanone building blocks.
Several biocatalytic approaches for the preparation of optically pure methyl 3
oxocyclohexanecarboxylates (S)-, (R)-1 and 3-oxocyclohexanecarbonitriles (S)-, (R)-2 have been
successfully demonstrated. Screening of reaction-focused enzyme collections was used to
identify initial hits using three enzymatic strategies. Reaction optimization and scale-up enabled
the production of chiral intermediates for route scouting efforts on scales of up to 100 g. The
enzymes applied in these processes (lipases, enoate reductases and nitrilases) have been shown to
be robust catalysts for drug manufacturing and represent a green alternative to conventional
methods to access these chiral cyclohexanone building blocks.
Biocatalysis, Enoate Reductases, Nitrilases, Lipases, Active Pharmaceutical
Ingredients.